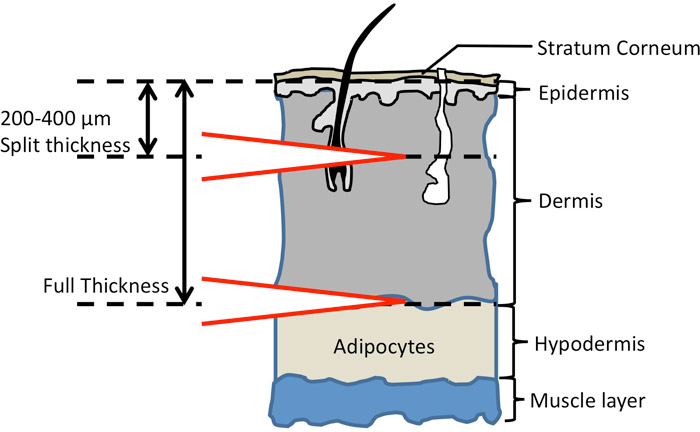
8.5 Dermal Absorption
Soil particles containing PAHs can adhere to skin and lead to exposures by dermal absorption. Although this guidance focuses on oral bioavailability, dermal absorption of PAHs can be important for assessing potential cancer risks, both systemically and at the site of contact on the skin. Using standard USEPA default exposure assumptions for oral and dermal exposures to soil, together with the current oral cancer slope factor for the index PAH, BaP, the ingestion route of exposure accounts for 72% and the dermal route of exposure accounts for 28% of the calculated cancer risk from systemic exposure due to direct contact with PAHs in soil. Currently, toxicity criteria for site-of-contact risk from dermal exposure to PAHs have not been established. If these values are available in the future, they should be considered in a dermal risk assessment. Initial data suggest that some of the same factors (desorption mechanisms) that control oral bioavailability of PAHs from soil are likely to affect dermal absorption as well (Xia, Gomez-Eyles, and Ghosh 2016; Peckham et al. 2016; USACE 2016).
8.5.1 In Vitro Dermal Studies of Absorption from Soil
Unlike the in vivo studies, the in vitro methodologies are generally based on established methodologies for evaluating dermal absorption of organic compounds such as the Organisation for Economic Co-operation and Development (OECD) Guideline for the Testing of Chemicals: Method 428. In vitro dermal absorption studies provide information on chemical flux across the skin barrier (chemical mass per square centimeter per hour; for example, ng/cm2-hr), which serves as the basis for calculation of a dermal absorption factor.
8.5.2 Elements of an In Vitro Dermal Study
In vivo dermal exposure studies are complex and the variations in testing conditions have added to the confusion regarding dermal bioavailability of PAHs. The elements that must be addressed when designing an in vitro dermal absorption study include the skin model used and its preparation prior to testing, the receiving fluid used, the experimental design, and the preparation of the soils tested. These element are discussed separately in the following sections.
8.5.2.1 Skin models and preparation.▼Read more
8.5.2.2 Receiving Fluid.▼Read more
8.5.2.3 Experimental Design.▼Read more
8.5.2.4 Test Soil Preparation.▼Read more