3 Technical Background
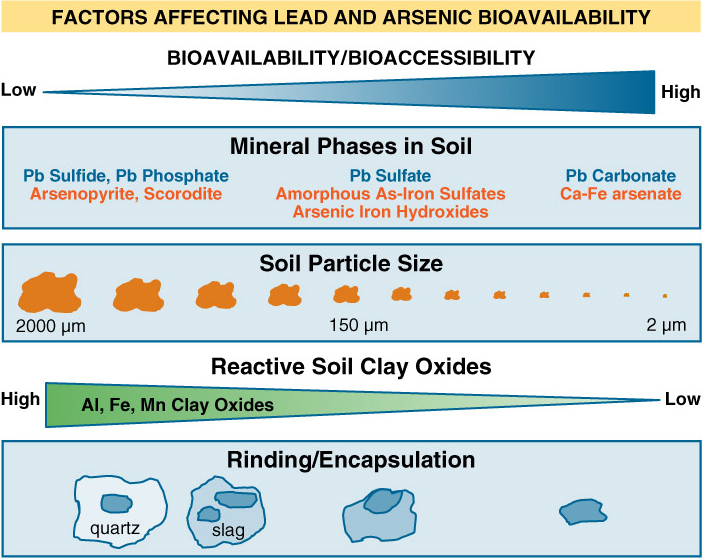
In this section is presented for soil and the properties of soil that influence bioavailability. The bioavailability of a contaminant in soil can vary widely depending on soil- or site-specific factors. Depending on the contaminant and site conditions, a variety of soil characteristics influence bioavailability, such as mineralogy, grain size, and soil organic matter. For metals, bioavailability is affected by mineralogy (form of the metal in the soil), soil particle size, and other factors that influence solubility. For organic chemicals, bioavailability is primarily influenced by sources of contamination and soil organic matter. Other important, site-specific factors include co-contaminants, historical site usage, and weathering of contaminants. Details of the specific source and soil characteristics that affect bioavailability are discussed in the chemical-specific chapters on lead, arsenic, and PAHs.
3.1 Soil Mineral Phases
Metals are often present within or adsorbed to mineral phases in soil. These minerals may be the initial source of elevated metals in the soil or may have formed within the soil from other metals sources.
3.2 Soil pH, Organic Matter, and Reactive Clay Minerals
Soil pH, organic matter and reactive clay minerals affect the bioavailability of contaminants in soil.
3.3 Soil Particle Size
Soil particle size also can affect the solubility of chemicals such as metals from soil, and thus their bioavailability.