6.1 Fate and Transport
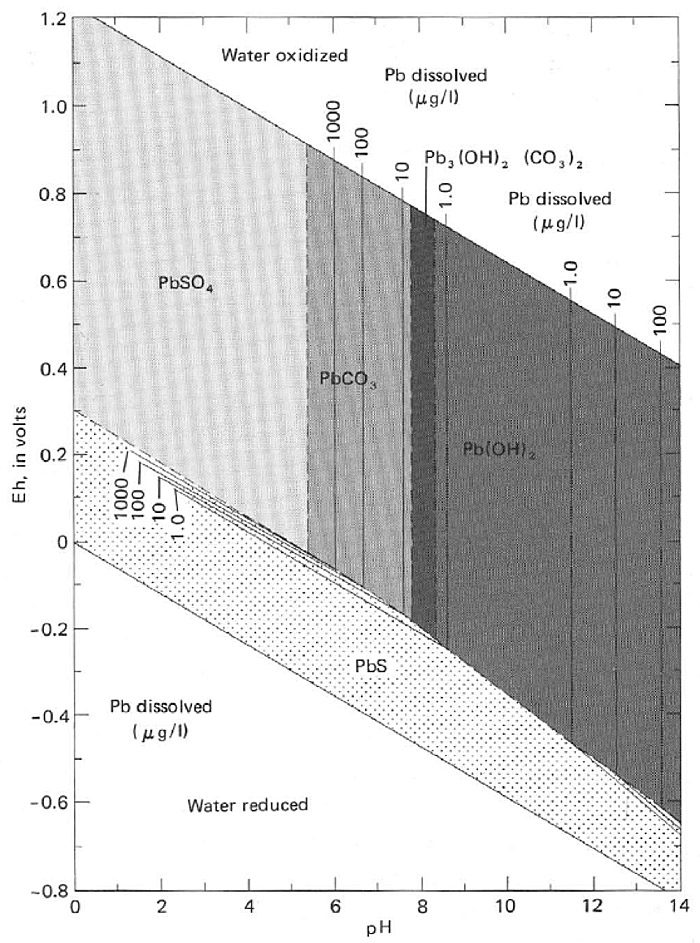
Soluble lead species in soil are subject to various reactions with the soil matrix that ultimately limit environmental transport. Many aqueous inorganic lead species exist in soil solution due to hydrolysis reactions, but are all cationic; freely dissolved lead is a divalent [+2] metal cation. Dissolved cations in most soils are subject to pH-dependent electrostatic attraction to negatively charged soil surfaces, such as with the functional groups of humic acids in soil organic matter (SOM) and onto the surface (and within the lattice) of a variety of secondary aluminosilicate clay minerals. These electrostatic reactions are generally considered relatively weak and exchangeable, whereas covalent (sometimes considered “irreversible”) reactions, in the case of lead, can occur with (1) the surfaces of amorphous iron and manganese (and to a lesser extent aluminum) oxides within a specific pH range (dependent on the specific metal oxide sorbent) and (2) by formation of very specific, inner-sphere complexes with SOM.