8.3 Influences of Soil on Bioavailability of PAH
Bioavailability of PAHs in soil is thought to be largely related to the partitioning of PAHs within the soil matrix, although in some recent studies partitioning alone could not explain differences in PAH uptake in swine orally exposed to different soils (James et al. 2016; Peters, Wickstrom, and Siciliano 2016). PAH partitioning in soils is dictated by the chemical and physical characteristics of the source of PAHs, chemical and physical properties of the soil matrix, and age of the release of PAHs to the environment.
8.3.1 Contamination Source and Matrix
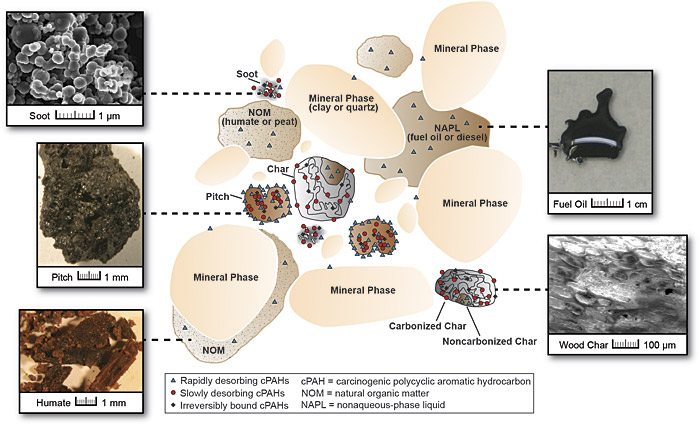
The source of PAHs must be considered when determining whether bioavailability assessments are likely to benefit risk management decisions. PAH source materials provide a matrix that can affect bioavailability. For example, PAHs released within black carbon type sources such as soot, chars, coal, or coke are associated with reduced bioavailability due to sorption. Nonaqueous phase liquids (NAPLs) sources such as petroleum products, fuel residues, creosote, or coal tar may also be associated with reduced bioavailability, but to a lesser degree (Ruby et al. 1996).
8.3.2 Geochemical and Physical Properties of Soil
Much research is available on the properties and sorptive capacities of soils and sediments as geosorbents for hydrophobic organic compounds (HOCs), including PAHs. Soil properties generally have less impact on PAH bioavailability than the source characteristics, but the inorganic and especially organic materials in soils and sediments also can have a significant effect. Generally, properties that control the sorptive capacity for hydrophobic organic compounds (HOCs) are the most important properties to consider.
8.3.3 Effects of Aging Contamination in Soil/Sediment on PAH Bioavailability
PAHs in soil and sediment undergo a weathering process over time that changes the overall chemical composition and can increase the sequestration of PAHs within the soil/sediment media (Alexander 1995, 2000; Northcott and Jones 2001; Luo et al. 2012). With time, PAHs in the rapidly desorbing fraction degrade, volatilize, or slowly diffuse into more sorptive and inaccessible phases within the soil matrix. Thus, the bioavailability and toxicity of PAHs remaining in soil and sediment may decrease over time.