1.2 Background
The National Research Council (NRC) has recognized the increasing value of risk assessments (2009):
Risk assessment has become a dominant public policy tool for making choices, based on limited resources, to protect public health and the environment. It has been instrumental to the mission of the [USEPA] as well as other federal agencies in evaluating public health concerns, informing regulatory and technological decisions, prioritizing research needs and funding, and in developing approaches for cost-benefit analysis.
Assessment of the risk of human exposures to chemical contaminants in the environment generally follows the framework set forth by the NRC (NRC 1983). USEPA’s human health risk assessment web page also includes information about risk assessment. Figure 1-1 illustrates the risk assessment process in the context of risk management.
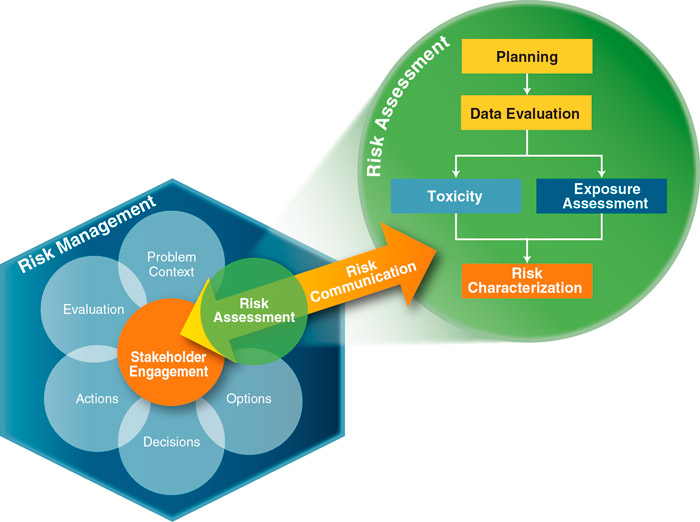
Figure 1‑1. Risk assessment process.
Source: ITRC RISK-3, Adapted from (Commission 1997).
The ITRC (2015) guidance describes the importance of using risk assessment as part of the whole process for understanding contaminated sites and making decision about what risk management is necessary:
Risk assessment is an integral component of risk management [for contaminated sites because it] provides a scientific and defensible rationale to support decisions for the protection of human health and the environment. Risk assessment is interconnected with risk communication and other components within the interactive, decision-making process for risk management (ITRC (2015)).
Bioavailability considerations are part of both the dose-response assessment and exposure assessment components of the risk assessment process; see the discussion of relative oral bioavailability for more information. Understanding the bioavailability of the contaminants in the soils at a site also supports risk-based decision making.
1.2.1 Bioavailability of Contaminants in Soil for Human Health Risk Assessment
The National Academy of Sciences defined “bioavailability” as the “individual physical, chemical, and biological interactions that determine the exposure of plants and animals to chemicals associated with soils and sediments” (NRC 2003).
Contaminant bioavailability has implications for a broad range of receptors, in both human health and ecological risk assessment. Similarly, bioavailability considerations may be relevant to assess potential risks from diverse environmental media. This guidance specifically addresses the bioavailability of chemicals in soil as part of a human health risk assessment, with a focus on incidental ingestion of soil. This guidance describes the state of the science for incorporating bioavailability considerations into the human health risk assessment process, including the appropriate use of bioavailability, and potential methods to estimate bioavailability, such as in vitro bioaccessibility tests. For additional information about human health risk assessment (see ITRC RISK-3).
1.2.1.1 Human Exposure Pathways for Soil
1.2.1.2 Human Exposure Pathways for Homegrown Produce
1.2.2 Bioavailability for Contaminated Sediments
1.2.3 Bioavailability for Ecological Receptors


