11.4 Resolution Copper Mining LLC (RCML) West Plant Site, Superior, Arizona
Contact: Joey Pace
Organization: Arizona DEQ
Email: [email protected]
Case Study prepared by Golder and Associates, Redmond, WA, (425) 883-0777
11.4.1 Site Description and Conceptual Site Model
A historical smelter stack operated from 1924 to 1972 by the Magma Copper Company is located within privately held land at the future location of the Resolution Copper Mine, operated by Resolution Copper Mining LLC (RCML). Rio Tinto has been the operator of RCML and has owned the Magma Copper Company mining property since 2004. The soil surrounding the smelter stack was characterized and evaluated in a human health risk assessment, and site-specific soil remediation levels (SSSRLs) were developed, in accordance with Arizona Administrative Code (AAC) R18-7-206, under the regulatory authority of the Arizona Department of Environmental Quality (ADEQ) Voluntary Remediation Program (VRP).
The selected remedial action for the smelter-affected soil (SAS) is removal and placement of soils above the SSSRLs as a portion of the mass grading fill needed in a tailings impoundment. This action is part of the site’s permitted closure plan. Closure activities at this facility are scheduled under the permit to begin during 2016.
The 1,780-acre site was divided into five land areas for evaluation, based on contaminant concentrations, topography, and accessibility. Roughly 200 soil samples were collected and analyzed, and from these data it was determined that the constituents of concern (COCs) for the SAS are arsenic, copper, and lead. The maximum observed soil concentrations of arsenic, copper, and lead were 6,990 milligrams per kilogram (mg/kg), 259,000 mg/kg, and 2,070 mg/kg, respectively. The highest observed constituent concentrations were in the Industrial Area North (IAN) and Industrial Area South (IAS) land areas, which are located closest to the stack (Figure 1), and are the focus of this case study. The three other land areas are the Silver King Wash, the Eastern Mountain Front (EMF), and the Northern Mountain Front (NMF), located to the northwest, east, and northeast of the industrial areas, respectively.
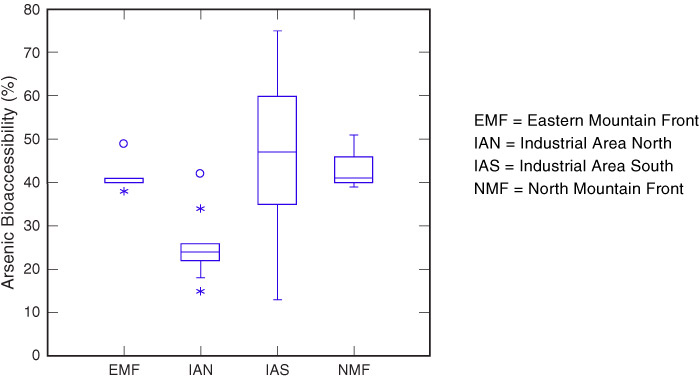
Land use for the entire West Plant Site is industrial, since it will be reused for mining, and public access in all of the land areas is illegal and restricted. The conceptual site model for the SAS assessed potential risks related to exposure to SAS via ingestion, inhalation, and dermal contact for industrial workers (adults only) and illegal trespassers (adults and teenagers). An initial sensitivity analysis showed that arsenic oral bioavailability was one of the more sensitive parameters in the calculation of arsenic risk. A default arsenic bioavailability value of 40%, based on literature studies, was approved by ADEQ for use in the development of SSSRLs. RCML elected to collect site specific data to better understand the range and variability of arsenic bioavailability across the site.
Figure 11-7 shows an overview of the West Plant site areas addressed in this case study.
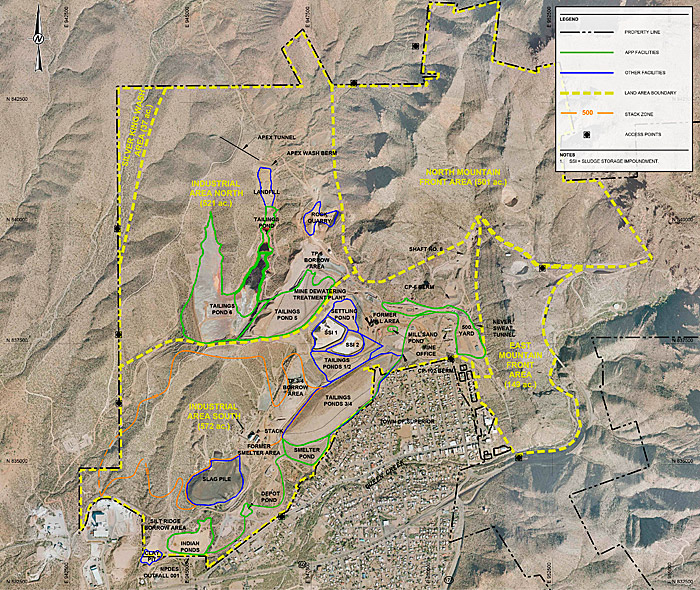
Figure 11‑7. West Plant site land areas.
Source: Courtesy Golder and Associates.
11.4.2 Methodology Used for Evaluating Bioavailability
11.4.3 Calculated Bioavailability of Arsenic in Soils
11.4.4 How Did Bioavailability Results Affect Site Decisions?